Question 2: What are the main features of the thermal radiations from a black body? Discuss Plank’s quantum theory and its importance in Physics.
ANSWER
Definition of Black Body
A blackbody is an ideal substance that absorbs all radiations falling on it.
A good approximation to a blackbody is some solid substance having a narrow opening. When a radiation enters through the opening, it is reflected repeatedly by the interior walls of the cavity. With each strike on the wall, a portion of energy is absorbed till the full absorption of the radiation. Similarly, when the walls of the cavity are heated to a high temperature, it emits all kind of radiations. Sometimes they find a chance to come out of the narrow opening. Such radiations are called blackbody radiations.
Main features of the black body radiations
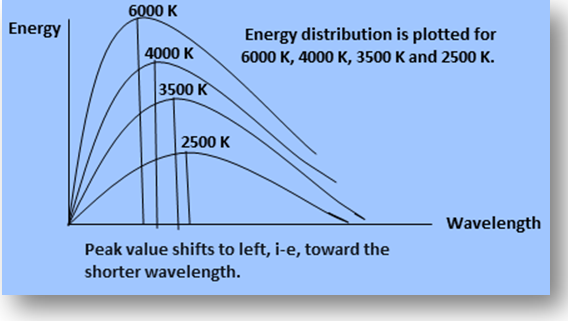
(1) Energy radiated from a blackbody varies with the wavelength and temperature. With the increase in temperature, emission of energy also varies. Also the peak of the energy distribution shifts to shorter wavelengths. This shift of the peak of energy distribution obeys the following equation.
λm T= constant
The value of this constant is 2.9 x 10-3 mK. This relation is called Wien’s displacement law. Here λm is the wavelength at the peak of the curve.
(2) At a particular temperature, total energy E radiated per second per square meter over all wavelengths is directly proportional to the fourth power of absolute temperature T.
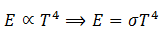
This relation is called Steffen-Boltzmann law.
(3) Raleigh and Jeans argued that the blackbody emits thermal radiations, which are reflected back and forth by the walls of the blackbody to form a system of standing waves. Based on this law they developed a formula for the energy distribution of blackbody radiations as
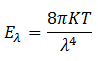
However, this law failed to explain the energy distribution of blackbody radiations. The curve obtained by them was fit with the experimental curve for longer wavelengths only. For shorter wavelengths, it could not agree with the experimentally obtained curve.
Planck’s Quantum Theory (Plank’s law)
In 1900, Planck introduced the quantum theory. The curve obtained by Planck was fit with the experimental curves both for shorter as well as longer wavelengths. According to Plank’s quantum theory, the blackbody radiations are not coming out continuously but in the form of packets or bundles of energy. These packets or bundles of energy are called photons or quanta. Similarly, the energy of each quantum is directly proportional to the frequency of radiation. If ‘f’ is the frequency of radiation, then the energy E of the quanta is given as
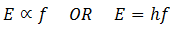
‘h’ is the Planck’s constant and its value if 6.63 x 10-34 J s.
Planck’s quantum theory can be summarized as follow;
- Each atom of the walls of the cavity behaves like a tiny electromagnetic oscillator, emitting and absorbing electromagnetic waves.
- The energy of the oscillator is En= nhf, where n is the quantum number and n = 1,2,3, …
- Oscillators do not radiate energy continuously. They emit energy in the form of quanta.
- As long as the oscillator is in a particular quantized state, its energy remains constant.
The energy distribution formula obtained by Plank is
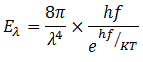
Planck was awarded with Noble Prize for this achievement in 1918.
Importance of Planck’s Quantum Theory
- Planck’s quantum theory has played an important role in the birth of Modern Physics. Einstein used Planck’s quantum theory to explain the photoelectric effect in 1905. Other unexplained facts like Compton effect can also be explained with this theory due to Planck.
- Quantum theory was used to explain line spectrum of Hydrogen atom.
In short, Planck’s quantum theory led to the development of the concepts of Modern Physics.

Pingback:photoelectric-effect-failure-of-classical-physics-success-of-photon-concept – msa
Pingback:einsteins-postulates-and-the-results – msa
Pingback:long-questions-ch-18-p12 – msa