Question 11: State Carnot theorem about the characteristics of a Carnot engine.
ANSWER
Carnot Theorem
A real heat engine can never be more efficient than a Carnot Heat Engine provided both operate between the same two heat reservoirs.
Consider two heat reservoirs at different temperatures so that one acts as HTR and the other as LTR. Let a heat engine operates between these two reservoirs. Then the efficiency of this heat engine will be maximum if it follows the Carnot cycle. Its efficiency will be less if it follows any processes other than cannot cycle.
Now the efficiency of a Carnot engine is
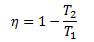
This indicates that the efficiency of a Carnot engine depends upon the difference of the temperatures of the reservoirs. For a high temperature difference, the value of T2/T1will be less and hence the term (1- T2/T1) will be large. Therefore, the efficiency η will also be greater. Thus the efficiency of a Carnot Engine will be increased by raising the temperature of HTR or lowering the temperature of LTR or doing both at the same time. Thus the efficiency is a function of two temperatures at which heat is supplied or rejected in the cycle and is independent of the nature of the working substance.
It should be noted that a temperature of 0 K cannot be attained and, therefore,the efficiency of a heat engine cannot be 100%.
