Question 3: What are the main features of photoelectric effect? Discuss the failure of classical physics and success of photon concept in explaining the effect.
ANSWER
Photoelectric Effect
Electrons are emitted from the surface of metal under the influence of electromagnetic radiations. This phenomenon is called photoelectric effect.
Explanation: Hertz first observed this effect in 1887. The experimental arrangements consist of an evacuated glass tube with a metallic cathode C and a metal anode A. Cathode C and anode A are connected to a variable voltage source along with a galvanometer as shown.
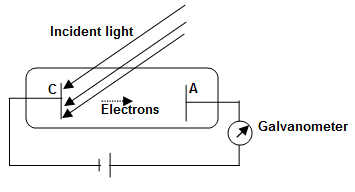
When the apparatus is kept in dark, galvanometer G shows no current. However, when monochromatic light of an appropriate wavelength is made to fall on cathode C, the galvanometer detects a flow of current. The electrons are emitted by cathode C and collected by anode A by moving across the tube between C and A. When the voltage V between C and A increases, the current Ip also increases until a saturation stage is reached (a saturation stage is one when all electron emitted by C are collected by A).
Maximum Kinetic Energy
Maximum K.E of the electrons can be determined by reversing the polarity of the electrodes, i-e, making C positive and A negative. Then we observe how many photo electrons reach A through the tube. Because only those electrons will reach plate A who have kinetic energy greater than the energy of the field. By increasing the reverse polarity of C and A, a stage will reach when no photo electron will reach plate A and the galvanometer will show no current flow. This potential difference is called stopping potential V0. Thus the work done in stopping the most energetic electron will be equal to the maximum K.E of the photoelectron.
(K.E)max = V0e
Main features of photoelectric effect
Following are the main features observed in the photoelectric effect:-
- Photoelectrons are emitted only when the frequency of the incident light is equal to or greater than the threshold frequency of the metal. In such a case the number of photoelectron is proportional to the intensity of the incident beam. Thus photoelectric current Ip is directly proportional to the intensity of the incident light.
- Meeting the condition of f greater than or equal to f0, photoelectrons are instantly emitted. If f<f0, no photoelectron is emitted even after long time of shining the metal surface.
- Maximum K.E of photoelectron depends upon the frequency of incident light. K.E of photoelectron is independent of the intensity of the incident of the electromagnetic radiation.
Failure of classical Physics
- Classical wave theory suggests that the phenomenon of the photoelectric effect can occur at all frequencies of the incident light. The experimental fact is that photoelectric effect occurs only when the frequency of the incident light is greater than or equal to the threshold frequency of that metal.
- Classical wave theory suggests that the K.E of the photoelectron depends upon the intensity of the incident radiation. The experimental fact is the K.E of the photoelectron depends upon the frequency of the incident light and not the intensity.
- Classical wave theory suggests that the emission of photoelectron should take long time to occur. The experimental fact is the emission of photoelectron occurs instantly when the light of proper frequency falls on the metal.
Therefore, we can conclude that the classical theory cannot explain the photoelectric effect.
Photon concept and success of explaining photoelectric effect
Einstein extended Planck’s theory of quantization to electromagnetic waves and successfully explained the phenomenon of photoelectric effect. Energy possessed by each photon is E = hf … (1)
Here h is the Planck’s constant and f is the frequency of light. When this photon falls on the surface of the metal, it collides with an inner electron. It transfers the energy to the electron and itself disappears. Now part of this energy is used to eject the electron from the surface of the metal and part as the K.E of the electron. The energy required to eject the electron from the metal is called work function of the metal, φ.
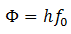
Here f0 is the threshold frequency for the metal surface. Therefore, we conclude that
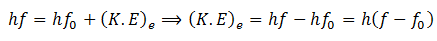
In addition, we know that
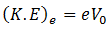
So equating the RHS of the above equations
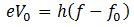
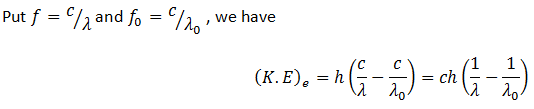
Einstein was awarded noble prize in 1921 for this explanation of photoelectric effect.
Clearly, if energy of the photon is less than the work function of the metal, no electron is emitted. If the energy of the photon is equal to the work function of the metal, electron is ejected but it has no kinetic energy.

Pingback:compton-effect-compton-shift – msa
Pingback:Features of Black body radiations and Planck’s quantum theory … msa – msa
Pingback:mcqs-ch-18-p12 – msa
Pingback:long-questions-ch-18-p12 – msa