Question 5: An electron and proton are accelerated from rest through the same potential difference. Which particle has the longer wavelength? Explain.
ANSWER
We know the de’ Broglie wavelength associated with a moving particle is λ=h/p. Now
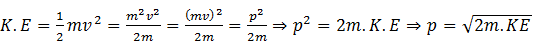
Put this value in the de’ Broglie wavelength equation given above.
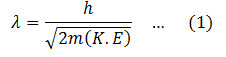
Now, when a charge q is accelerated through a potential difference V0, the K.E it gains is,
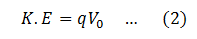
Put this in equation (1)
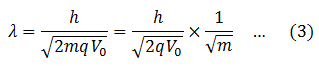
Now the charge (q) on electron and proton is same. They are accelerated under the same potential difference, so V0 is also the same. Plank’s constant, h, is already a constant. Therefore, the first term on the RHS of equation (3) is constant. Therefore,
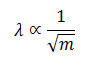
Thus the wavelength associated with the motion of the particle is inversely proportional to the square root of the mass of the particle. If the mass is heavier, the wavelength is smaller and vice versa.
In the given case, the mass of proton is heavier than the mass of electron. (Mass of proton is approximately 1836 times heavier than the mass of electron). Therefore, the wavelength associated with proton will be smaller and the wavelength associated with electron will be longer.

Pingback:objects-in-dark-rooms-are-not-seen – msa
Pingback:sq-ch18-p12 – msa
Pingback:temperatures-of-red-and-blue-stars – msa