A thermodynamic system can exert force of
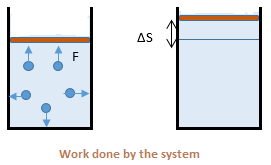
the surrounding to push or displace it through some distance. For example, the gas molecules hitting the boundary of the container and displacing it. Consider the adjacent figure. The sum of all
F.ΔS over the whole boundary is the work done by the system on its surroundings. By convention, work done by the system is considered positive and work done on the system as negative.
Pingback:Theory of Thermodynamics – msa