Question 10: What is meant by Carnot Cycle and Carnot Engine?
ANSWER
Carnot Cycle
A number of processes after the completion of which the properties of the system returns to the original state is called a cycle.
The operating cycle of a Carnot cycle consists of the following four processes.
1. Isothermal Expansion
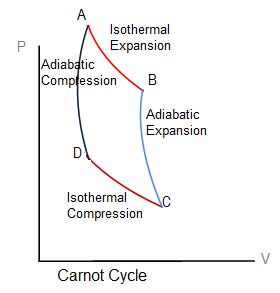
Consider a Carnot heat engine, which consists of a gas cylinder with perfectly insulating walls and perfectly conducting base, having an insulated, weightless and frictionless piston.
Let the initial pressure, volume and temperature of the system are P1,V1 and T1, respectively. Place the cylinder on a high temperature reservoir (HTR). The gas absorbs some amount of heat (+ Q1). With the heat thus provided, the gas expands and the volume increases and pressure decreases. Thus, the temperature remains constant. After the absorption of heat (+Q1) and the resultant expansion, the state of the gas is described with pressure P2, volume V2 and temperature T1(which is constant). Therefore, the process is isothermal represented by the isotherm A-B in the figure.
2. Adiabatic Expansion
The cylinder is then placed on a heat insulation stand so that the heat can neither enter nor leave the system. The gas is allowed to expand under these conditions and let the new volume is V3. With this expansion, the temperature of the gas in the cylinder falls down. Say, it is T2 now. Similarly, the pressure decreases from P2 to P3. The process is an adiabatic expansion and represented by the adiabat B-C in the figure.
3. Isothermal Compression
The gas cylinder is now placed on cold reservoir (LTR) at temperature T2. The gas is compressed by increasing the load on the piston. Heat (-Q2) is rejected by the gas to the LTR and in this way the temperature remains constant. However, the volume decreases from V3 to V4 and pressure goes from P3 to P4. The process is an isotherm shown by C-D in the figure.
4. Adiabatic Compression
The gas cylinder is placed on the insulating stand and compressed to the initial stage of P1, V1 and T1. The process is represented by the adiabat D-A in the figure.
The four processes thus complete a Carnot cycle.
In one complete cycle, a Carnot heat engine performs a net amount of work ΔW, and absorbs a net amount of heat Q1 – Q2 in the process, represented by the area ABCDA.
Efficiency
Efficiency is defined as,

Since Q and T are proportional, therefore,
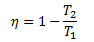
This equation says that the efficiency of a heat engine can never reach to 100%, because T2/T1must have some positive numerical value and when subtracted from 1 will make the efficiency η < 1.

Pingback:index-cq10-p11