Question 1: State and explain Coulomb’s law. Do include the case when the charges are placed in dielectrics. Discuss how the unit of charge coulomb is defined?
ANSWER
Coulomb’s law
French mathematician, Coulomb, studied the force between two point charges in 1785 and gave this law which states that, “two stationary charges q1 and q2 attract or repel each other with a force which;
- Is directly proportional to the scalar product q1q2 of the magnitudes of charges.
- Is inversely proportional to the square of the distance r between them.
- Acts along the line joining the centers of the charges.”
Mathematically,
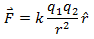
Here k is the constant of proportionality. Its value depends upon the system of units used and the medium between the two charges. The ‘electrostatic constant’ k, as it is sometimes called, is expressed in terms of permittivity of the free space ε0.
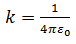
Experimentally determined value of ε0 is 8.85 × 10-12 C2 N-1 m-2. Therefore, putting the values of π and ε0,
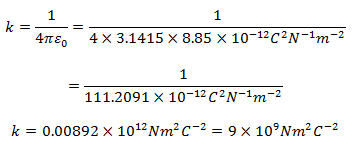
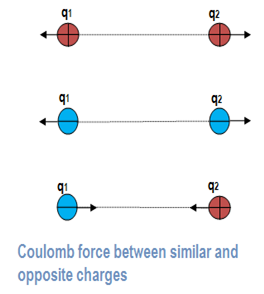
The direction of the force is shown by a unit vector along the line joining the two charges. For like charges the product q1 q2 is positive and the force between them is repulsive. For unlike charges, the product q1q2 is negative and the force between the charges is attractive.
It should be noted that both charges exert equal and opposite forces on one another. Therefore, if F21 is the force exerted by charge q1 on charge q2 and F12 is the force exerted by charge q2 on charge q1, then
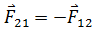
When the charges are placed in a dielectric medium
It has been found experimentally that when an insulator or dielectric is placed between the two point charges, the force between them decreases. In such cases, Coulomb’s force is given by,
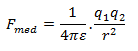
Here ε is the permittivity of the medium.
Relative permittivity
The ratio of the permittivity of the dielectric ε with the permittivity of vacuum ε0 is called relative permittivity of the dielectric or dielectric constant, εr. Therefore,
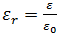
Note that εr has no dimensions.
| Permittivity is the measure of the degree to which a medium can resist the flow of charge. It tells us how the magnitude of the force of the charges is affected when they are placed in a certain medium. Relative Permittivity is the permittivity of the medium compared with the (i-e, divided by) permittivity of the free space. Its value ranges from 1 to 4000 but for most material it doesn’t exceed 10. |
ε is always greater than ε0 and therefore εr is always greater than 1.
So the electrostatic force in a medium of relative permittivity is given by,
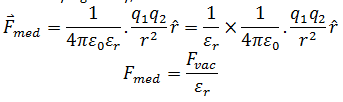
So the dielectric force reduces as compared to the force between the same two charges in vacuum.
Definition of Coulomb; Unit of charge
Coulomb, the unit of charge, can be defined by considering the electrostatic force the charges exert on one another. In SI, the unit of charge is coulomb and is defined as coulomb is that much charge which exerts on an equal and similar charge a force of repulsion of 9 × 109 newtons placed in air at a distance of 1 meter. In terms of current, the coulomb is defined as it is the charge transported by a steady current of one ampere in one second.
