Question 3: State and explain First Law of Thermodynamics.
ANSWER
Definition
The law states that every thermodynamic system possesses a state variable ‘U’ called the internal energy.
Explanation
The law is, in fact, a particular form of conservation of energy when applied to heat energy. It explains that when heat energy is converted into mechanical energy or when mechanical or some other form of energy is converted to heat energy, the total energy remains the same.
Mathematical expression
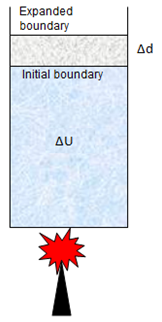
Consider an ideal gas enclosed in a cylinder with a movable piston. If some amount of heat energy ΔQ is added to the system, it increases the internal energy ΔU of the system and at the same time work ΔW is done by the system on the surrounding by pushing the system up. So
ΔQ = ΔU + ΔW —– (1)
Here,
ΔQ is positive if heat enters the system.
ΔQ is negative if heat leaves the system.
ΔW is positive if work is done by the system.
ΔW is negative if work is done on the system.
ΔU is positive if temperature of the system increases.
ΔU is negative if temperature of the system decreases.
Mathematical expression of the first law of thermodynamics indicates that the change in the internal energy of the system is equal to the energy flowing in as heat minus the energy flowing out as work. So the change in the internal energy ΔU is the energy retained by the system. This energy changes the kinetic and potential energy of the system.
This should be noted that the change in internal energy of the system depends upon the initial and final states and not on the path followed to carry out this change. If UAand UB are internal energies in the initial and final states,respectively, then,
ΔU = UB – UA
Put this value in equation (1),
UB – UA = ΔQ – ΔW
In case the system undergoes a cyclic process, i-e, it attains the same state after undergoing some process,then,
UA = UB
or UB – UA = 0
Therefore, ΔQ – ΔW = 0
or ΔQ = ΔW
So, in cyclic process, the heat energy absorbed by a system is used to do some useful work.
