Problem 7: If there are N0 = 6.02 * 1023 atoms in 4 gm of helium. What is the mass of one helium atom?
Solution
Given data: No of helium atoms = 6.02 * 1023
Mass of the above no of atoms = 4 g
Required: Mass of 1 atom of helium atom
Now
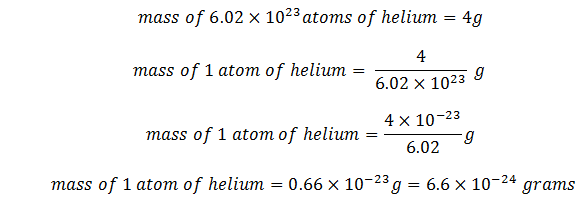
This is the required result.
Pingback:numerical-problems-on-measurement-physics-11 – msa