In a single isolated atom, electrons remain in definite energy levels.
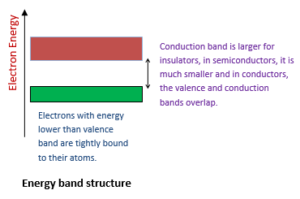
However, in solids, the atoms are close to each other and the energy levels of the outermost orbit electrons are affected by the neighboring atoms. The electrons of the outermost orbits of one atom experiences an attraction from the neighboring nuclei. The energy levels of electrons are changed into bands (i-e, groups) of closely spaced levels with large energy gaps between them. The spaces between these energy gaps are called forbidden energy states (or gaps) and the bands as permissible energy levels. In solids, large number of atoms and the energy levels divide into bands of closely spaced levels with large energy gaps between them. These energy states are discrete (separate) in nature but are so close to each other that they appear in the form of a continuous energy band.