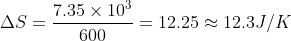Solution
When 1 kg of lead is melted, 2.45 × 104 J of heat is added to it. Therefore, when 3 × 102 g = 3 × 10-1 kg of lead is melted 2.45 × 104 × 3 × 10-1 = 7.35 × 103 J of heat ΔQ is added to it. Similarly, the temperature is 327° C = 327 + 273 = 600 K. So, apply formula ΔS = ΔQ/T,