At constant temperature, volume of a given mass of a gas is inversely proportional to the pressure applied on it.
Mathematically,
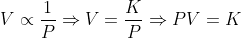
(At constant temperature)
This means that for constant temperature, the product of pressure and volume of a gas always remains constant.
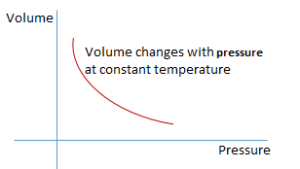
If temperature is kept constant and a graph is drawn between pressure and volume at different values respectively, the graph is a curve.
Pingback:States of Matter: Gases, Comprehensive Questions… msa