Theory: When water is at a height 854 m, it possesses some potential energy. When it falls down, this energy is converted to kinetic energy. The kinetic energy converts to heat to increase the temperature of water. So in fact, the heat energy will be equal to the P.E at the top.
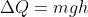
Similarly, if ΔQ is the heat, m is the mass and ΔT is raise in temperature of a substance, then
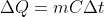
Compare the two equations,
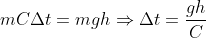
Put values,
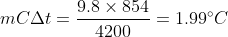
So, final temperature will be = initial temperature at top + change in temperature at bottom
Final temperature = 20° + 1.99°C = 21.99°C ≅ 22°C