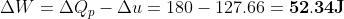Given Heat given at constant pressure, ΔQp = 180 J
γ = 1.41
Required (i) Increase in the internal energy, ΔU
(ii) Work done by the ideal gas, ΔW
Formulae: 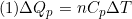 (2)
(2) 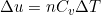 (3)
(3) 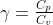
Calculation: Divide (1) by (2),
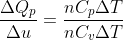
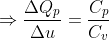
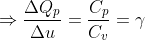
Put values of ΔQp and γ from the given data,
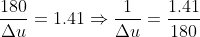
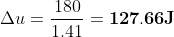
To calculate the work done, apply first law with this value of Δu.