Question 3: What do you understand by the terms normal state, excited state, excitation energy, ionization energy.
ANSWER
Normal State
Atom has the tendency to retain its minimum energy state. The minimum energy state of the atom is known as normal or ground state.
Excited State
When an atom absorbs energy from the surrounding and one or more of its electron move to higher energy state, the atom is said to be in the excited state. In the excited state the atom is unstable and returns to the ground state in a while.
Excitation Energy
Energy required to move an electron from its ground state to an excited state is known as excitation energy.
Explanation
Under normal conditions, the atom remains in the ground state. Ground state is the lowest energy state. When the energy of the electron is increased, it jumps to a higher energy state, called the excited state.
We know that the energy of an electron in the nth orbit is En = -(E0/n2) where E0 = 13.6 ev is the energy of the electron in the ground state, representing n = 1. The other higher values of n are known as excited states.
The first excitation energy of the electron comes from when it jumps from the ground state n = 1 to n = 2. Thus the first excitation energy is
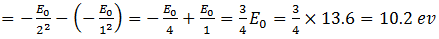
So to raise an electron in the ground state to n = 2, 10.2 ev of energy is required. For raising to 3, 4, 5 etc orbits, substitute n = 3, 4, 5 etc and the corresponding excitation energy will be obtained.
Ionization Energy
It is defined as the minimum energy required to remove an electron in the ground state to an infinite energy state. From Bohr’s atomic theory, we know that the energy of an electron in the nth orbit is En = -(E0/n2). In the ground state, the calculated value of the energy of the electron is -13.6 eV. In the infinite state, therefore, the energy of the electron is
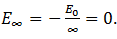
When the electron posses no energy, it means it is not bound to the nucleus and is free to move. When its energy further increases, it is completely knocked out leaving the atom ionized. So the ionization energy is
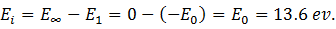

Pingback:x-rays-properties-and-uses – msa
Pingback:bohr-postulates-about-h-atom – msa
Pingback:long-questions-ch19-p12 – msa